上海金畔生物科技有限公司为生命科学和医药研发人员提供生物活性分子抑制剂、激动剂、特异性抑制剂、化合物库、重组蛋白,专注于信号通路和疾病研究领域。
Lometrexol (Synonyms: DDATHF)
Lometrexol (DDATHF) 是一种抗嘌呤类抗叶酸 (antifolate) 药,可抑制甘氨酰胺核糖核苷酸甲酰基转移酶 (GARFT) 的活性,但不会引起可检测水平的 DNA 链断裂。Lometrexol 可以进一步抑制嘌呤从头合成,导致异常的细胞增殖,凋亡 (apoptosis) 和细胞周期停滞。Lometrexol 具有抗癌活性。Lometrexol 还是一种有效的人丝氨酸羟甲基转移酶 1/2 (hSHMT1/2) 抑制剂。
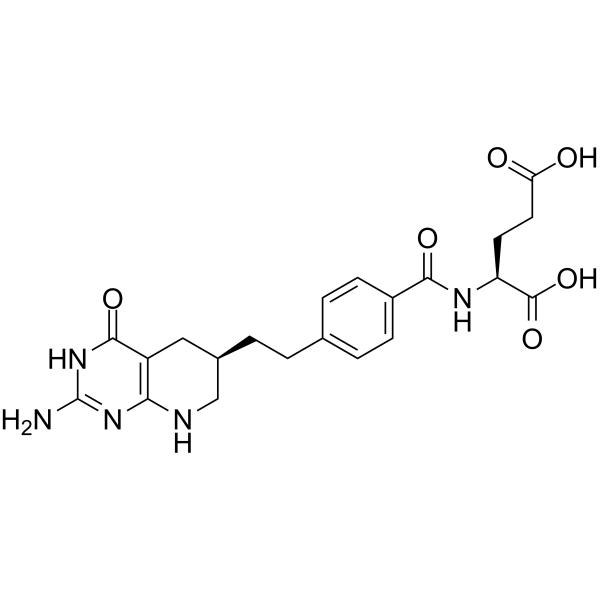
Lometrexol Chemical Structure
CAS No. : 106400-81-1
| 规格 | 价格 | 是否有货 | |
|---|---|---|---|
| 10 mM * 1 mL in DMSO | ¥8097 | 询问价格 & 货期 | |
| 1 mg | ¥2750 | 询问价格 & 货期 | |
| 5 mg | ¥8300 | 询问价格 & 货期 | |
| 10 mg | ¥13950 | 询问价格 & 货期 | |
* Please select Quantity before adding items.
Lometrexol 的其他形式现货产品:
| 生物活性 |
Lometrexol (DDATHF), an antipurine antifolate, can inhibit the activity of glycinamide ribonucleotide formyltransferase (GARFT) but do not induce detectable levels of DNA strand breaks. Lometrexol can further inhibit de novo purine synthesis, causing abnormal cell proliferation and apoptosis, even cell cycle arrest. Lometrexol has anticancer activity[1][2]. Lometrexol also is a potent human Serine hydroxymethyltransferase1/2 (hSHMT1/2) inhibitor[3]. |
IC50 & Target |
GARFT[1] |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 体外研究 (In Vitro) |
Lometrexol (DDATHF) binds tightly to GART, resulting in a rapid and prolonged depletion of intracellular purine ribonucleotides[2]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. Cell Viability Assay[2]
Cell Cycle Analysis[2]
|
||||||||||||||||
| 体内研究 (In Vivo) |
Lometrexol (DDATHF; i.p.; 15-60 mg/kg; on gestation day 7.5) increases the rate of embryonic resorption and growth retardation in a dose-dependent manner[1]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only.
|
||||||||||||||||
| Clinical Trial |
|
||||||||||||||||
| 分子量 |
443.45 |
||||||||||||||||
| Formula |
C21H25N5O6 |
||||||||||||||||
| CAS 号 |
106400-81-1 |
||||||||||||||||
| 中文名称 |
洛美曲索 |
||||||||||||||||
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
||||||||||||||||
| 储存方式 |
Please store the product under the recommended conditions in the Certificate of Analysis. |
||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 40 mg/mL (90.20 mM) * “≥” means soluble, but saturation unknown. 配制储备液
|
||||||||||||||||
| 参考文献 |
|
所有产品仅用作科学研究或药证申报,我们不为任何个人用途提供产品和服务
